Despite tremendous research efforts, cancer is a leading cause of mortality worldwide. According to World Health Organisation (WHO) the global cancer burden is estimated to 19.3 million new cases and 10 million deaths in 2020. Breast cancer (BRC) is the most common cancer among women, affecting 2.2 million women each year, and the greatest cause of cancer-related deaths among them. In 2020, 685000 women died from BRC, which makes up approximately 15% of all cancer deaths in females. Cancer statistics in Serbia is also wearisome: around 36000 new cancer patients and 20000 deaths are reported each year. Similar to global situation, BRC is the leading cause of both morbidity (29.9%) and mortality (18.9%) in women suffering from cancer in our country. The cancer burden continues to rise worldwide, exerting immense physical, emotional and financial strain on affected patients, families, societies and healthcare systems. Importantly, cancer incidence is expected to increase by 47% in the next 20 years, which will likely be concomitant with increased mortality. Common breast cancer treatments, including radiation, chemo- and endocrine therapy, are often accompanied by undesirable side effects, which may lead to incomplete treatment, reduced quality of life (QoL), cancer recurrence or increased risk of other chronic diseases.
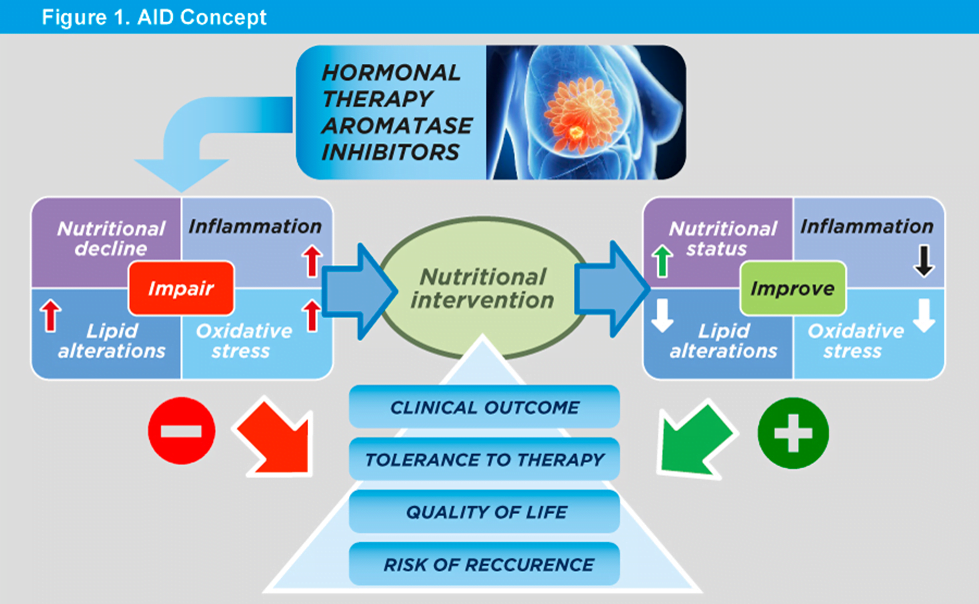
Anti-cancer adjuvant therapies, such as endocrine therapy with aromatase inhibitors (AI) in hormone-dependant BRC, have several adverse effects: muscle and joint pain, osteoporosis, inflammatory diseases, cardiovascular events and sexual dysfunction. Due to the long duration of adjuvant AI therapy (5-10 years), patient tolerability issues may arise, and thereby compromise treatment compliance, adherence and therapeutic outcome. Both the treatment and disease may lead to a decline in nutritional status (malnutrition, sarcopenia, osteopenia, obesity) which is associated with poor response to treatment, increased risk of recurrence and non-BRC-related morbidity and mortality. Many of these complications could be prevented by proper nutrition.
Our proposed project AID addresses these challenges. It is designed as a randomized clinical trial with tailored diet and supplements in a duration of 4 months and a comparison among two intervention and one control groups. The implementation of this project will help BRC patients to prevent the decline in nutritional status, to better tolerate the therapy, decrease side effects and risks of comorbidities, and thereby improve clinical outcomes and survival rates.